WELCOME TO OUR PARKINSON'S PLACE!
I HAVE PARKINSON'S DISEASES AND THOUGHT IT WOULD BE NICE TO HAVE A PLACE WHERE THE CONTENTS OF UPDATED NEWS IS FOUND IN ONE PLACE. THAT IS WHY I BEGAN THIS BLOG.
I COPY NEWS ARTICLES PERTAINING TO RESEARCH, NEWS AND INFORMATION FOR PARKINSON'S DISEASE, DEMENTIA, THE BRAIN, DEPRESSION AND PARKINSON'S WITH DYSTONIA. I ALSO POST ABOUT FUNDRAISING FOR PARKINSON'S DISEASE AND EVENTS. I TRY TO BE UP-TO-DATE AS POSSIBLE.
I AM NOT RESPONSIBLE FOR IT'S CONTENTS. I AM JUST A COPIER OF INFORMATION SEARCHED ON THE COMPUTER. PLEASE UNDERSTAND THE COPIES ARE JUST THAT, COPIES AND AT TIMES, I AM UNABLE TO ENLARGE THE WORDING OR KEEP IT UNIFORMED AS I WISH. IT IS IMPORTANT TO UNDERSTAND I AM A PERSON WITH PARKINSON'S DISEASE. I HAVE NO MEDICAL EDUCATION,
I JUST WANT TO SHARE WITH YOU WHAT I READ ON THE INTERNET. IT IS UP TO YOU TO DECIDE WHETHER TO READ IT AND TALK IT OVER WITH YOUR DOCTOR. I AM JUST THE COPIER OF DOCUMENTS FROM THE COMPUTER. I DO NOT HAVE PROOF OF FACT OR FICTION OF THE ARTICLE. I ALSO TRY TO PLACE A LINK AT THE BOTTOM OF EACH ARTICLE TO SHOW WHERE I RECEIVED THE INFORMATION SO THAT YOU MAY WANT TO VISIT THEIR SITE.
THIS IS FOR YOU TO READ AND TO ALWAYS KEEP AN OPEN MIND.
PLEASE DISCUSS THIS WITH YOUR DOCTOR, SHOULD YOU HAVE ANY QUESTIONS, OR CONCERNS. NEVER DO ANYTHING WITHOUT TALKING TO YOUR DOCTOR FIRST..
I DO NOT MAKE ANY MONEY FROM THIS WEBSITE. I VOLUNTEER MY TIME TO HELP ALL OF US TO BE INFORMED.
I WILL NOT ACCEPT ANY ADVERTISEMENT OR HEALING POWERS, HEALING FROM HERBS AND ETC. UNLESS IT HAS GONE THROUGH TRIALS AND APPROVED BY FDA. IT WILL GO INTO SPAM.
THIS IS A FREE SITE FOR ALL WITH NO ADVERTISEMENTS
THANK YOU FOR VISITING! TOGETHER WE CAN MAKE A DIFFERENCE!
TRANSLATE
Friday, April 1, 2011
Thursday, March 31, 2011
Experts Call for Stricter Regulations in Clinical Trials of New Drugs
Clinical Research News
Experts Call for Stricter Regulations in Clinical Trials of New Drugs
Last year that was illustrated when Dendreon's prostate drug Provenge was approved in April by the U.S. Food and Drug Administration because clinical trials went well, and five months later Eli Lilly infamously had to pull its Alzheimer candidate drug semagacestat because test subjects using it were actually getting worse -- putting both in the news.
"There seems to be two narratives people can handle: Experiments are dangerous and you shouldn't take part, or experimentation is the engine for the next great wonder drug," said Alex John London, an associate professor of philosophy at Carnegie Mellon University who is the co-author of an article in the current issue of the journal PLoS Medicine that looks at this and related issues with clinical trials. "What we need is a more even set of expectations."
That's important, Mr. London said, because if people believe there are only two possible outcomes at either extreme, they might not be willing to participate in clinical studies.
"If people don't participate, studies don't happen," he said.
Mr. London and his colleague, Jonathan Kimmelman, an associate professor in the biomedical ethics unit at McGill University in Montreal, co-authored the article titled, "Predicting Harms and Benefits in Translational Trials: Ethics, Evidence and Uncertainty."
But while arguing that our expectations of clinical trials need to be more realistic and informed, Mr. London and Mr. Kimmelman also believe that the trials themselves need to be proposed, and run, more rigorously.
The article -- which Mr. London sees as "taking part in a wider conversation" with researchers, funders, review boards and other stakeholders -- goes right at what they see as some of the problems in both pre-clinical animal-based trials, and the transitional, first-in-human clinical trials where so many proposed drugs fall apart.
They both point out the problems they see in clinical trials, and make proposals for how they could be corrected -- with potentially far-reaching implications in drug research.
"I thought they raised incredibly important points," said Nancy Davidson, director of the University of Pittsburgh Cancer Institute and UPMC Cancer Centers. "I think Kimmelman and London are right: This black and white, either really good or really bad theme, isn't the answer. "
The co-authors were motivated to write the article, Mr. Kimmelman said, because "only rarely do the effects seen in animal studies translate into human studies."
That is, that even though researchers regularly find success with a proposed drug during research on animals, only infrequently do researchers achieve similar success with human test subjects.
They note in their paper that one study showed, for example, that only 5 percent of proposed cancer drugs that enter trials are eventually licensed.
"Drug discovery is hard," Mr. London said. "I don't think there's any way of getting around that."
And that gets to a central theme to their work, Mr. Kimmelman said: "What we're really trying to do here is check the expectations associated with major clinical findings."
That has important implications not only for possible human test subjects -- who need to have a better understanding of potential outcomes -- but for organizations that provide research funding "and who would want to move their resources into the most promising areas," Mr. London said.
Still, they believe clinical trial outcomes might be improved were it not for two main problems they have found.
The first is that most animal studies don't use the same generally accepted methods used in human trials -- such as randomization and blinded outcome assessment -- to prevent bias by the researcher.
They note that one recent analysis of animal studies found that only 12 percent of those studies used random allocation -- where animals are randomly assigned to take the proposed drug or to get a placebo -- and only 14 percent used blinded outcome assessment -- where a researcher doesn't know which animals were getting the drug and which were getting the placebo.
These are important because "researchers have an inherent bias in wanting to see a positive outcome" from the studies, Mr. Kimmelman said.
Moreover, Mr. London said that the routine use of randomization and blind testing in animal studies should increase the efficiency of the drug development process, "if for no other reason than that it enables us to better distinguish effects caused by new interventions from outcomes that are simply artifacts of the way we have set up the experiment."
Sean Savitz, a stroke researcher and associate professor of neurology at the University of Texas Medical School at Houston, said: "What they're getting is what we've been trying to get at in the stroke field -- that studies with animals are not being done as well as they could be."
The second problem they worry about is that they believe that researchers don't look widely enough at other related research in making their predictions about the possible outcome once they begin human trials.
They believe that most researchers, when predicting the outcome of their study, only consider other studies involving the particular agent, or drug, they are testing. The researchers don't look at agents that might be different but that work on the same pathway, they argue. Mr. London and Mr. Kimmelman refer to this process as "evidential conservatism."
Howard Mann, a program associate in the Division of Medical Ethics at the University of Utah School of Medicine, said this point made by Mr. London and Mr. Kimmelman is troubling.
"What should be alarming is the persistent conduct of pre-clinical research that will not yield clinically relevant information because researchers are not aware of, or have not made sufficient attempts to identify, the negative results of prior completed relevant research," Mr. Mann said in an e-mail statement about the article.
As an example of this, the authors presented two tables with their article: One shows the negative outcomes of eight randomized trials of anti-amyloid drug candidates for Alzheimer's disease, similar to Eli Lilly's semagacestat; and the other shows the negative outcomes of seven randomized trials for neuroprotective agents and/or transplanted tissue strategies to combat Parkinson's disease.
"If consistently neuroprotective strategies have failed, we're suggesting that people should ratchet down their expectations," Mr. Kimmelman said.
Researchers might be doing that on their own, Mr. London said, but they should also include their findings, and lower expectations, in their proposals to the committees that have to review the results of an animal trial before allowing it to move on to human trials.
Including such information in proposals could result in some research not being funded, Mr. Kimmelman acknowledged. But it could also push researchers to "come back and explain how their work is going to overcome previous results."
Ultimately, Mr. London said: "We want to make sure we design our trials as well as possible so we can learn from them -- even if it is a failure in outcome.
"It's not that we learn from every failure, but it's that when they are well designed, we can learn much more from them."
Source Publication: Pittsburgh Post-Gazette
Neurologix Gene Therapy Helps Parkinson’s Patients Walk, Move in Study
- Clinical Research News
Neurologix Gene Therapy Helps Parkinson’s Patients Walk, Move in Study
The inserted gene acts like a factory, pumping out one of the brain chemicals lacking in people with the disorder to rebalance substances that stimulate and inhibit activity, said Michael Kaplitt, a founder of Neurologix and co-creator of the therapy. The study was published today in the journal Lancet Neurology.
The therapy appeared safe and seemed to improve motor control, compared with subjects who received a placebo through a sham surgical procedure, said Jon Stoessl, director of the Pacific Parkinson’s Research Centre at the University of British Columbia in Vancouver. Stoessl, who wasn’t involved in today’s findings, said the finding should be evaluated in larger trials.
“This is the first controlled trial to show a significant improvement in the active group of a gene therapy trial,” Stoessl said in a telephone interview. “It appears promising and merits further investigation.”
Neurologix, which has no marketed products, will apply to the FDA to begin an expanded trial next year that may be used to win regulatory approval, Marc Panoff, the company’s chief financial officer, said yesterday in a telephone interview.
Seeking Investors
The company needs to raise $30 million to $40 million to conduct the study and is talking to both investors and potential partners from the pharmaceutical industry to obtain funding, Panoff said.Walter Liskiewicz, 60, a retired oral surgeon who has been living with Parkinson’s for 18 years, said he was in “terrible” shape before taking part in the study.
“I wiggled all the time, I had trouble walking, life sucked,” Liskiewicz said.
After being treated in July, 2009, Liskiewicz started noticing slow, steady improvement, he said in a telephone interview yesterday. Now he walks a mile or two once or twice a week and can carry groceries into his house.
“This is not a neuroprotective treatment, which would be the holy grail,” said Blair Ford, a professor of clinical neurology at Columbia University in New York. “This is aimed at ameliorating the immediate symptoms of Parkinson’s, not reversing its course.”
Gene Therapy Revival
About 1.5 million people in the U.S. have Parkinson’s, according to the Parkinson’s Disease Foundation. People with the neurological disease gradually lose neurons that make the brain chemical dopamine. The lack of dopamine leads to increasing activity in a part of the brain called the subthalamic nucleus, which influences movement.While no gene therapy has yet been approved by the U.S. Food and Drug Administration, recent successes have revived a field that was almost ended when a patient in such a trial died in 1999. Cambridge, Massachusetts-based Bluebird Bio, a closely held company, is developing therapies for a vision disorder and blood conditions including beta thalassemia and sickle cell disease after clinical trials reported positive results. Bluebird is backed by Genzyme Corp. (GENZ), also in Cambridge, and by venture capital firms.
Early-stage Parkinson’s patients now are treated with medications including Boehringer Ingelheim GmbH’s Mirapex, GlaxoSmithKline Plc’s Requip and a generic medication called levodopa. All aim to increase dopamine in the brain.
Effectiveness Wanes
These treatments generally lose effectiveness after several years. Patients may then get a treatment called deep-brain stimulation, which shoots electrical current into the subthalamic area of the brain. While the procedure helps many patients, they must have batteries and wires installed in their bodies, said Neurologix’s Kaplitt, a neurosurgeon at Weill Cornell Medical College in New York.In the Neurologix therapy, an ultra-thin wire is inserted through the skull and passes through brain tissue to reach the subthalamic area. The gene, carried by a harmless virus, journeys into brain cells to ramp up production of the chemical GABA, which limits excess activity to reduce abnormal movements.
The therapy was first tested in 12 patients in a 2007 study. While those findings were encouraging, no control group was used so researchers couldn’t be certain about the findings.
In the latest study, 22 patients received an injection with the gene, called GAD. For comparison, 23 people underwent a sham procedure in which surgeons pretended to drill holes in their skulls and imitated sounds of the actual procedure.
Catheter Inserted
A catheter designed by Minneapolis-based Medtronic Inc. (MDT) to lock in place after being inserted allowed patients to leave the operating room and have the gene injected in a recovery room, making the procedure less invasive and more affordable. Afterward, the catheter was removed and a small cap was used to cover the hole in the skull.Both groups of patients showed improvement on a scale that rates stiffness, tremors, speed of motion and other symptoms. After six months, patients who got the treatment improved by 8.1 points, or 23 percent, while the placebo patients had a 4.7 point, or 11 percent, improvement, Kaplitt said yesterday in a telephone interview. Some patients did even better, he said.
“All but two of the patients in the treatment group had some improvement and half of them had dramatic improvement,” Kaplitt said. There were no serious adverse events related to the treatment, he said.
The therapy didn’t appear to alleviate the cognitive problems, depression and impaired balance many patients suffer, Stoessl said.
Gene therapy has several advantages over deep-brain stimulation, said Kaplitt.
“It’s a single operation instead of two, patients don’t need general anesthesia and no hardware is left in the brain,” he said.
Source Publication: Bloomberg
Wednesday, March 30, 2011
Tuesday, March 29, 2011
National Parkinson Foundation
Education For Patients
NPF Helpline
NPF is pleased to announce the launch of 1-800-4PD-INFO (473-4636), our newest initiative to provide information (in English and Spanish) to people with Parkinson's disease, their families, friends and healthcare providers. Services available include up-to-date information about the disease in general, emotional support, and referrals to health professionals and community resources. For more information, visit our Helpline page: www.parkinson.org/helpline.NPF Resources
NPF maintains a wide range of free consumer educational resources to help people with Parkinson's and their caregivers to actively manage the disease and become their own effective health advocates. Materials are distributed to thousands of patients and caregivers through our Centers of Excellence and Care Centers, as well as our local chapters. Educational resources provided by NPF are available in a variety of formats, from online versions of brochures and booklets, to archived webcasts of live conference events, and videos of Parkinson’s disease experts explaining topics important to know.Publications
NPF continues to offer print copies of our popular ‘Rainbow Books’, the widely respected nine-volume Parkinson's education series focused on issues critical to patients. Patients, families and professionals alike consider these books essential for education about PD, ranging from introductory information to more in-depth material that can be kept and reread many times over.In addition to NPF's newsletter, the Parkinson Report, NPF offers an extensive seven-volume Spanish-language series and brochures in eight languages. Our newest publication is titled ’10 Early Warning Signs of Parkinson’s Disease’ and reviews ten signs that may mean someone has early stage PD and should talk to their doctor. All print booklets, brochures and newsletters are free of charge and may be requested by calling 1-800-4PD-INFO.
What are the possible complications of Parkinson’s disease?
What are the possible complications of Parkinson’s disease?

Depression, anxiety - sometimes depression may occur before other Parkinson’s symptoms appear. According to the National Parkinson’s Foundation, USA, it is thought that up to 50% of patients with PD experience a mood disturbance at some point during their illness.
Sexual dysfunction - some patients experience a drop in libido (sex drive). Sexual dysfunction affects more males than females.
Sleep - patients often wake up during the night. A significant number of individuals with Parkinson’s disease find it hard to fall asleep. Being sleepy and falling asleep during the day is also common.
Urinary incontinence or retention - some patients may leak while others find it hard to pee properly. Sometimes this may be due to medications used to treat Parkinson’s disease.
Medications - some Parkinson’s disease medications can cause:
- Hypotension when standing up (blood pressure drops upon standing from seated or lying position)
- Involuntary twitching/jerking of arms and legs
- Hallucinations
- Drowsiness
- Obsessive compulsive behavior
What Are The Treatment Options For Parkinson’s Disease?
What Are The Treatment Options For Parkinson’s Disease?

Treatment approaches include medication, surgery, general lifestyle modifications (rest and exercise), physical therapy (UK: Physiotherapy), support groups, occupational therapy and speech therapy.
Medication
Medication - as most Parkinson’s symptoms are caused by low levels of dopamine in the brain, most drugs are aimed at either replenishing dopamine levels, or mimicking its action - dopaminergic drugs do this. Dopaminergic medications reduce rigidity (muscle stiffness), improve speed, help with coordination, and lessen tremor (shaking). Taking dopamine itself does not help, because it cannot enter the brain.- Levodopa - the most effective Parkinson’s drug; is absorbed by the nerve cells in the brain and turned into dopamine. It is taken orally, in tablet or liquid form. Levodopa is combined with carbidopa to create Sinemet, a combination drug. Carbidopa prevents the levodopa from being destroyed by enzymes in the digestive tract; it also reduces levodopa side effects, such as nausea, vomiting, fatigue and dizziness. In the UK and the rest of Europe benserazide may be combined with levodopa (Madopar).
- As Parkinson’s disease progresses the effects of levodopa may wear off and the doctor may have to increase the dosage. Increased dosage also raises the risk of developing side effects, which may include confusion, delusions, hallucinations, compulsive behavior, and dyskinesia (involuntary movements). Reducing the dosage will usually help with side effects, but with the risk that parkinsonism increases.
- Dopamine agonists - these drugs mimic the effects of dopamine in the brain. The neurons react as they would to dopamine. Although not as effective as levodopa, dopamine agonists last longer and help reduce the waning effect of levodopa. They are usually prescribed in tablet form, but may also be taken by injection, or as a skin-patch. Examples include pramipexole (Mirapex), ropinirole (Requip), rotigotine (Neupro), and apomorphine (Apokyn).
- Side effects are similar to those of carbidopa-levodopa. The risk of developing compulsive behaviors, such as compulsive gambling and hypersexuality are greater.
- Monoamine oxidase-B inhibitors (MAO B inhibitors) - an alternative to levodopa. Examples include selegiline and rasagiline. MAO B inhibitors work by blocking the effects of monoamine oxidase-B (MAO B) in the brain. Monoamine oxidase-B destroys dopamine - by blocking MAO B the dopamine can last longer in the brain. MAO B inhibitors have a smaller effect than levodopa. MAO B inhibitors can be used in combination with levodopa or dopamine agonists. There is a risk of serious interactions with some medications used for treating depression, as well as some narcotics.
- Side effects of selegiline may include:
- Dizziness
- Dry mouth
- Headaches
- Nausea
- Stomach pain
- Strange and/or vivid dreams
- Side effects of rasagiline may include:
- Conjunctivitis
- Dizziness
- Fever, with joint and muscle aches (flu-like)
- Headache
- Indigestion
- Neck pain
- Runny nose
- Stomach pain
- COMT (catechol O-methyltransferase) inhibitors - this medication blocks the enzyme that breaks down levodopa, hence prolonging the effect of carbidopa-levodopa therapy.
- Anticholinergics - used for controlling tremor (shaking). Examples include trihexyphenidyl and benztropine (Cogentin). Some patients may find that the side effects are much greater than the slight benefits. Side effects may include urine retention, severe constipation, nausea and dry mouth. Male patients with an enlarged prostate have a higher risk of developing urine retention.
- Antivirals - may be used on its own during early-stage Parkinson’s disease. May also be used alongside carbidopa-levodopa therapy later on. Side effects include ankle edema (swelling) and skin discoloration. An example of this drug is amantadine (Symmetrel).
- Physical therapy (UK: physiotherapy) - exercise is crucial for maintaining function. Physical therapy can help the patient improve mobility, range of motion, as well as muscle tone. Physical therapy cannot stop the progression of Parkinson’s disease, but it can help the patient cope and feel better. The physical therapist can help relieve muscle stiffness and joint pain through movement and exercise. A qualified physical therapist (UK: physiotherapist) can help the patient improve balance and gait.
- Speech therapy - according to the National Health Service (NHS), UK, approximately half of all Parkinson’s patients experience communication problems, such as slurred speech and poor body language. A speech and language therapist can help with the use of language and speech. Patients with swallowing difficulties may also be helped by a speech therapist.
- Occupational therapy - an occupational therapist can pinpoint everyday life problems and help work out practical solutions. Examples include getting dressed, or getting the shopping done.
Surgery
- Deep brain stimulation - a surgical procedure used to treat several disabling neurological symptoms, such as tremor, rigidity, stiffness, slowed movement and walking difficulties.
- An electrode is implanted deep inside the brain, where movement is controlled. A pacemaker-like device (neurostimulator), which controls the amount of stimulation delivered by the electrode, is placed under the skin in the upper chest. A wire travels under the skin and connects the neurostimulator to the electrode.
- Electrical impulses are sent from the neurostimulator, along the wire, and into the brain via the electrode. They interfere with the electrical signals that cause symptoms, effectively blocking them.
- Deep brain stimulation is generally used when the patient is in the advance stages of Parkinson’s disease, and has unstable medication responses.
- The procedure has some risks, including brain hemorrhage and infection. Patients who do not respond to carbidopa-levodopa therapy do not benefit from deep brain stimulation.
- Thalamotomy - the thalamus is destroyed (lesioned) or removed by cutting (ablated). The thalamus is a tiny part of the brain. The proecedure may help reduce tremor. Thalamotomy is rarely performed these days. It may be used for patients with tremor who have not responded to medication. The procedure does not improve slow movement, walking difficulties or speech problems.
- Pallidotomy - since the introduction of deep brain stimulation, this procedure is rarely done. The gobus pallidus, a part of the brain, may be overactive in patients with Parkinson’s disease, causing a different part of the brain which controls movement to become less active. The surgeon destroys a small part of the globus pallidus by creating a scar, resulting in less activity in that area of the brain, which in turn may help relieve movement symptoms, such as rigidity and tremor.
- Subthalamotomy - rarely performed these days. The subthalamus, a very small area of the brain, is destroyed.
Alternative Therapies
Alternative therapies - according to the National Health Service (NHS), UK, up to 40% of patients with Parkinson’s disease in the UK use some type of alternative therapy, such as massage, acupuncture or herbal remedies. Patients using herbal remedies and/or supplements should tell their doctor - some may interact with Parkinson’s medications.Nutrition
Nutrition - some patients with Parkinson’s disease suffer from constipation. A diet high in fiber, as well as adequate fluid consumption is important for reducing the number of incidences as well as severity of constipation.Postural (Orthostatic) Hypotension
Postural (orthostatic) hypotension - low blood pressure when changing position - is another problem experienced by some Parkinson’s disease patients. Doctors may advise an increase in salt and fluid intake, as well as avoiding products with caffeine in the evening, eating many small meals a day, and abstaining from alcoholic drinks.If the patient loses weight - a common problem with Parkinson’s disease - he/she may be referred to a dietitian.
Diagnosing Parkinson’s Disease

A GP (general practitioner, primary care physician), usually the first health care professional people see, will base diagnosis on the signs and symptoms, the patient’s medical history, as well as the results of a clinical examination.
Initially, when symptoms are mild during the early stages of Parkinson’s disease, a GP will find it hard to definitively diagnose the condition. If Parkinson’s is suspected, the GP will probably refer the patient to a specialist (neurologist).
It is vital that the doctor has experience with all the possible disorders than can masquerade as Parkinson’s disease.
As part of their medical history, the physician will need to know about any drugs the patient is/was taking, and also whether any close family members have/had Parkinson’s disease.
A neurological examination usually evaluates the patient’s walking, coordination, and some simple hand tasks. The doctor may also check the patient’s sense of smell. He/she may also prescribe a medication for Parkinson’s disease - if it helps symptoms, it may help find out whether the individual has the disease.
The following tests may be ordered:
- Blood test - usually to rule out any other condition, such as abnormal thyroid hormone levels or liver damage.
- MRI or CT scan - to check for signs of a stroke or brain tumor. If there is/was no stroke or brain tumor, most MRI or CT scans of people with Parkinson’s disease will appear normal.
- PET (positron emission tomography) scan - this imaging test may sometimes detect low levels of dopamine in the brain. As PET scans are expensive and not present in all hospitals, this option may sometimes not be available. PET is a highly specialized imaging technique which uses radioactive substances to create 3-dimensional colored images of those substances functioning in the body. Information about the body’s chemistry can be gained with a PET scan, which is not the case with other imaging techniques.
- Two of the four main symptoms must be present - for a neurologist to consider a Parkinson’s disease diagnosis, the patient must have two of the four main symptoms. They must be present over a specific period. The four main symptoms are:
- Tremor or shaking
- Bradykinesia - slowness of movement
- Rigidity (stiffness) of the arms, legs or trunk
- Postural instability - balance problems and possible falls
- Look at a detailed medical history of the patient
- Carry out a physical exam
- Check medications currently being taken, and those taken in the past
- Carry out a detailed neurological exam, during which the patient performs tasks to asses agility of legs and arms, muscle tone, gait, and balance.
- Usually, results of an exam are entered into a table, called United Parkinson’s Disease Rating Scale (UPDRS). UPDRS was created to comprehensively asses and document the examination of a patient with Parkinson’s disease, and be able to compare data entered with future follow up examinations, or to communicate data with other neurologists.
- Observe the patient’s response to Parkinson’s disease medications (drugs that stimulate Dopamine production or imitate it). An example of a Parkinson’s drug is levodopa.
- Two of the four main Parkinson’s symptoms have been present for some time
- Symptoms started on just one side of the body
- Tremor (shaking) is more evident at rest
- A Parkinson’s drug (e.g. levodopa) produces a strong, positive response
What Are The Causes of Parkinson’s Disease?
What Are The Causes of Parkinson’s Disease?

If the Dopaminergic cells in the brain are damage or perish, dopamine production goes down and the messages from the substantia nigra and the corpus striatum do not work properly. Parkinson’s disease signs and symptoms appear when four-fifths of these nerve cells are lost. As dopamine levels continue to drop, the signs and symptoms of Parkinson’s disease get worse.
Put simply:
- Parkinson’s disease is caused by the degeneration or destruction of dopamine-producing nerve cells (dopaminergic cells), which in turn makes it harder for the brain to control and coordinate muscle movement.
What Are The Risk Factors For Parkinson’s Disease?
What Are The Risk Factors For Parkinson’s Disease?

- Age - the older you get the greater the risk. Although Parkinson’s disease can affect young people, this is exceptional.
- Genetics - a person who has a close relative (brother, sister, mother, father) with Parkinson’s disease has a slightly higher risk of developing it himself/herself, compared to others. Even so, according to The Mayo Clinic, USA, the risk is still less than 5%.
- Gender - males are slightly more likely to develop Parkinson’s disease compared to females.
- Toxin exposure - individuals who have been exposed to some chemicals, such as carbon monoxide, herbicides or pesticides have a slightly higher risk of developing Parkinson’s disease, compared to other people.
- Some medications - such as antipsychotics used to treat severe paranoia and schizophrenia can cause Parkinsonism (symptoms that resemble Parkinson’s disease).
What Are The Signs and Symptoms of Parkinson’s Disease?
What Are The Signs and Symptoms of Parkinson’s Disease?

Parkinson’s disease causes problems with movement, cognitive problems, neurobehavioral problems, as well as sensory and sleep difficulties. The signs and symptoms usually begin gradually, slowly and often randomly (in no set order).
Each sufferer will be affected differently, with a unique set of symptoms. Patients also tend to respond differently to treatment. Symptom severity also varies enormously. Some patients may experience tremor (shaking) as their primary symptom, while others may not have tremors, but have balance problems. While the disease may develop slowly for some individuals, for others it progresses rapidly.
The four main signs and symptoms include slow physical movements (bradykinesia), shaking (tremor), muscle stiffness (rigidity) and postural instability (impaired balance and coordination). They are called the primary motor symptoms:
Primary motor symptoms:
- Bradykinesia (slowness of movement, slowed motion) - initiating movement, such as beginning to get up from a chair can become more difficult. The patient typically takes longer to carry out tasks. There is also a lack of coordination. The difficulty is not only with the execution of movement, but also with its planning and initiation. Bradykinesia is often tolerated by elderly patients, who think they are entering normal milestones of aging - such patients may eventually be diagnosed with PD later on, when other signs and symptoms develop.
- Resting tremor (shaking) - the characteristic shaking frequently starts in one hand, such as a back-and-forth rubbing of the thumb or forefinger (pill-rolling). Tremor may start in a foot or one side of the body, and less commonly in the jaw or face. Tremor is usually more likely to occur when that part of the body is resting - stress and/or anxiety may make the tremor more noticeable. However, substantial tremor is not always present in many patients. Other conditions may include tremor as one of their symptoms, such as multiple sclerosis, encephalitis (inflammation of the brain), or alcoholism. The presence of tremor does not necessarily mean the individual has Parkinson’s disease. According to the Parkinson’s Disease Foundation, USA, approximately 70% of people with Parkinson’s experience a slight tremor in the early stages.
- Rigidity (muscle stiffness) - the muscles feel stiff. Doing some everyday tasks may be troublesome, such as getting out of a chair, rolling over in bed, using body language appropriately, or making fine finger movements. Most commonly, stiffness occurs in the limbs and neck. It can be so severe that the range of movements is severely undermined. Sometimes there is pain.
- Posture and balance - there may be instability when standing, or impaired balance and coordination. These symptoms, combined with bradykinesia significantly increase the risk of falling.
- A tendency to stoop, to lean forward
- Cramping
- Drooling
- Fatigue
- Handwriting may be very small and cramped (micrographia)
- Impaired fine motor dexterity (fine finger movements)
- Impaired motor coordination
- Involuntary movements and prolonged muscle contractions (dystonia)
- Loss of facial expression - some individuals may appear uninterested (not animated) when speaking, while others stare fixedly with unblinking eyes.
- Sexual dysfunction
- Speech problems - the sufferer may have a softer voice, utterances may come out more rapidly or slowly, or in a monotone. There may be repeated words or slurring.
- Swallowing difficulties (dysphagia)
- The arms may not swing when walking
- Dementia - this may develop in the later stages of the disease. The patient may have memory and mental clarity problems. A person with Parkinson’s is six times more likely to develop dementia, compared to other people.
- Sleep problems - which may be worsened by medications for Parkinson’s disease. However, sleep problems are a core feature of the disease. The patient may be excessively sleepy during the day; there may be disturbances in REM (rapid eye movement) sleep, as well as insomnia.
- Constipation
- Depression
- Dysphagia (difficulty swallowing)
- Fatigue, tiredness, loss of energy
- Paresthesia - a sensation of tingling, pricking, or numbness of a person's skin (pins and needles)
- Reduced sensation of pain
- Reduced sense of smell
- Urinary incontinence (bladder weakness)
- Urinary retention (difficulty getting rid of urine)
What Is Parkinson’s Disease? What Causes Parkinson’s Disease?

Illustration of the Parkinson disease
by Sir William Richard Gowers
from A Manual of Diseases of the
Nervous System in 1886 (WikiMedia Commons)
Parkinson’s disease is both chronic and progressive. Chronic means long-term, while progressive means it gradually gets worse.
Parkinsonism is a neurological syndrome characterized by tremor, rigidity, postural instability, and hypokinesia (decreased bodily movement). A syndrome is the association of several clinically recognizable features, signs, symptoms, phenomena or characteristics that often occur together. Parkinson's disease is the most common cause of Parkinsonism. Put simply - Parkinsonism includes the signs and symptoms that resemble Parkinson’s disease.
While about 5% of individuals with Parkinson’s disease are under the age of 40 years, the majority are over 50. When signs and symptoms develop in an individual aged between 21 and 40 years, it is known as Young-onset Parkinson’s disease. Approximately 1 in every 20 patients diagnosed with PD is under 40 years of age. When signs and symptoms appear in people under 18 years of age, it is known as Juvenile Parkinson’s disease. It affects both sexes; males slightly more than females.
According to the National Institutes of Health (NIH), USA, approximately 500,000 Americans are affected by Parkinson’s disease; about 50,000 new diagnoses are made each year. The National Health Service (NHS), UK, estimates that about 120,000 people in the United Kingdom are affected.
As a significant number of elderly patient with early Parkinson’s disease symptoms assume that their symptoms may form part of normal aging and do not seek medical help, obtaining accurate statistics is probably impossible. There are also a several different conditions which sometimes have comparable signs and symptoms to PD.
PD is named after James Parkinson (1755-1824), an English apothecary surgeon, paleontologist, geologist and political activist. In his most famous work An Essay on the Shaking Palsy (1817), he was the first person to describe paralysis agitans, which eventually was named after him.
Parkinson’s disease belongs to a group of conditions called movement disorders. Movement disorders describe a variety of abnormal body movements that have a neurological basis, and include such conditions as cerebral palsy, ataxia, and Tourette syndrome. Parkinson’s disease results from decreased stimulation of the motor cortex by the basal ganglia, typically caused by insufficient formation and action of dopamine.
There is no current cure for Parkinson’s disease (April, 2010). Treatment focuses on alleviating symptoms. Sometimes treatment may include surgery.
According to Medilexicon's medical dictionary:
Parkinsonism is:
- A neurologic syndrome usually resulting from deficiency of the neurotransmitter dopamine as the consequence of degenerative, vascular, or inflammatory changes in the basal ganglia; characterized by rhythmic muscular tremors, rigidity of movement, festination, droopy posture, and masklike facies.
- A syndrome similar to parkinsonism. Some features seen with Parkinson’s disease that occur with other disorders (progressive supranuclear palsy) or as a side effect of certain medications (antipsychotic drugs).
Parkinson's Rehab Resource Center
APDA's National Resource Center for Rehabilitation, based at Boston University's Sargent College of Health and Rehabilitation Sciences, puts you in contact with a licensed physical therapist who can answer questions about exercise, provide information about programs in your area, and supply free educational materials about the benefits of exercise for people with PD.
The American Parkinson Disease Association (APDA) is the country’s largest grass roots organization serving the Parkinson’s community. It is a 501 © (3) not-for-profit organization and receives no governmental or public funding. Each year APDA contributes more than $2.5 million for research and another $2 million for direct patient and caregiver support through the generous support of individual and corporate donations. You can be a partner in easing the burden and finding a cure in a variety of ways while honoring a loved one or even helping to secure your own financial future.
Clue:Gene Mutation That Causes Parkinson's Disease
Researchers at Mount Sinai School of Medicine have discovered a way that mutations in a gene called LRRK2 may cause the most common inherited form of Parkinson's disease. The study, published online this month in the journal Public Library of Science, shows that upon specific modification called phosphorylation, LRRK2 protein binds to a family of proteins called 14-3-3, which has a regulatory function inside cells. When there is a mutation in LRRK2, 14-3-3 is impaired, leading to Parkinson's. This finding explains how mutations lead to the development of Parkinson's, providing a new diagnostic and drug target for the disease.
Using one-of-a-kind mouse models developed at Mount Sinai School of Medicine, Zhenyu Yue, PhD, Associate Professor of Neurology and Neuroscience, and his colleagues, found that several common Parkinson's disease mutations-including one called G2019S-disturb the specific phosphorylation of LRRK2.This impairs 14-3-3 binding with varying degrees, depending on the type of mutation.
"We knew that the LRRK2 mutation triggers a cellular response resulting in Parkinson's disease, but we did not know what processes the mutation disrupted," said Dr. Yue. "Now that we know that phosphorylation is disturbed, causing 14-3-3 binding to be impaired, we have a new idea for diagnostic analysis and a new target for drug development."
Dr. Yue's team also identified a potential enzyme called protein kinase A (PKA), responsible for the phosphorylation of LRRK2. Although the exact cellular functions disrupted by these changes are unclear, their study provides a starting point for understanding brain signaling that contributes to the disease. Recent studies have shown that 14-3-3 binds to other proteins implicated in inherited Parkinson's disease and has a neuroprotective function, and when the binding is impaired due to these mutations, the protection may be lost. The findings also demonstrate additional insight into the functional relevance of the LRRK2 and 14-3-3 interaction.
The presence of 14-3-3 in spinal fluid is already used as a biomarker for the presence of neurodegenerative diseases. Further applications of these findings could point to the use of 14-3-3 as a biomarker in testing for Parkinson's disease.
Dr. Yue's team at Mount Sinai includes Xianting Li, PhD, Associate Scientist, and Nina Pan, Associate Researcher; collaborators Brian T. Chait, PhD, and Qing Jun Wang, PhD, from the Rockefeller University; and Yingming Zhao, PhD, and Sangkyu Lee, PhD, from the University of Chicago. Dr. Yue's work is supported by grants from the National Institutes of Health, and The Michael J. Fox Foundation for Parkinson's Research.
Source:
Mount Sinai Medical Center
Also Included In: Neurology / Neuroscience
Article Date: 25 Mar 2011 - 10:00 PDT
CHEMOKINES IN PARKINSON'S DISEASE
CHEMOKINES IN PARKINSON'S DISEASE
Neuro-inflammation is often claimed to be a cause or contributor to the cause of Parkinson's Disease by damaging or interfering with the dopaminergic neurons (the cells involved in Parkinson's Disease). Neuro-inflammation is often a response of the Central Nervous System to injury. Chemokines play a role in the effect of inflammatory diseases. For more information go to Chemokines. So the levels of chemokines were compared in people with and
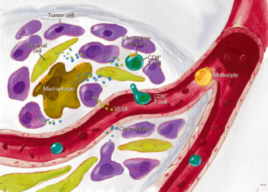 without Parkinson's Disease. The levels of the Chemokines CCL3, CCL11, CCL24, CXCL8 and CXCL10 were assessed. The levels of the chemokines were then related to the severity of Parkinson's Disease. Each person with Parkinson's Disease was assessed using three different measures of Parkinson's Disease. However, the researchers found no difference in the levels of chemokines between people with and without Parkinson's Disease. So chemokines are not indicators of Parkinson's Disease. Also, the idea that Parkinson's Disease is due to inflammation of the Central Nervous System was largely nullified.
without Parkinson's Disease. The levels of the Chemokines CCL3, CCL11, CCL24, CXCL8 and CXCL10 were assessed. The levels of the chemokines were then related to the severity of Parkinson's Disease. Each person with Parkinson's Disease was assessed using three different measures of Parkinson's Disease. However, the researchers found no difference in the levels of chemokines between people with and without Parkinson's Disease. So chemokines are not indicators of Parkinson's Disease. Also, the idea that Parkinson's Disease is due to inflammation of the Central Nervous System was largely nullified.THE WORST NON-MOTOR SYMPTOMS IN PARKINSON'S DISEASE
Although muscular movement is the most characteristic symptom of Parkinson's Disease, non-motor symptoms (those that do not concern muscular movement) are increasingly recognised as neglected aspects of Parkinson's Disease. The most common non-motor symptom has been found to be urinary. In order of frequency the most common symptoms are : autonomic dysfunction, particularly urinary and gastrointestinal
 symptoms (84%), mood (74%), fatigue (74%), daytime somnolence (65%), pain (56%), nocturnal sleep problems (55%), and psychosis (55%). Motor fluctuations (57%) and dyskinesia (43%) were associated with worse quality of life. Depression had the strongest association with a lower health related quality of life, then fatigue, thermoregulatory, gastrointestinal, cardiovascular autonomic function, daytime somnolence, and urinary problems. Whilst psychiatric problems are increasingly documented, many symptoms, particularly those perceived as embarrassing or unrelated, remain under reported.
symptoms (84%), mood (74%), fatigue (74%), daytime somnolence (65%), pain (56%), nocturnal sleep problems (55%), and psychosis (55%). Motor fluctuations (57%) and dyskinesia (43%) were associated with worse quality of life. Depression had the strongest association with a lower health related quality of life, then fatigue, thermoregulatory, gastrointestinal, cardiovascular autonomic function, daytime somnolence, and urinary problems. Whilst psychiatric problems are increasingly documented, many symptoms, particularly those perceived as embarrassing or unrelated, remain under reported. THE PREVALENCE OF EYE DISORDERS IN PARKINSON'S DISEASE
 information go to Dry Eye Syndrome. In Glaucoma, the optic nerve fibers become damaged. Small blind spots can occur, and can develop in to significant loss of vision. Most people with glaucoma do not notice the symptoms until they have significant loss of vision. For more information go to Glaucoma. A quarter of people with Parkinson's Disease have visual hallucinations. The visual hallucinations are usually due to an excess of dopaminergic drugs such as L-dopa in its various forms, or dopamine agonists. Visual hallucinations are not normally due to a problem with the structure of the eyes.
information go to Dry Eye Syndrome. In Glaucoma, the optic nerve fibers become damaged. Small blind spots can occur, and can develop in to significant loss of vision. Most people with glaucoma do not notice the symptoms until they have significant loss of vision. For more information go to Glaucoma. A quarter of people with Parkinson's Disease have visual hallucinations. The visual hallucinations are usually due to an excess of dopaminergic drugs such as L-dopa in its various forms, or dopamine agonists. Visual hallucinations are not normally due to a problem with the structure of the eyes. DISTINGUISHING PARKINSON'S DISEASE USING THE EYES
Eye Brain Tracker.
 The equipment consists of : a recording device for high-frequency, accurate eye movement measurement, two screens, an introductory computer application for behavioural testing, and an automatic test analysis application. When an eye movement exam is conducted, the tests are displayed on a screen. The subject receives instructions on how to take the tests. The subject's eye movements are recorded while he takes the test. Once the tests are completed, the examiner may conduct a thoroughly automatic analysis, with the option of manually adjusting the results to enhance quality. Based on this analysis, an eye movement report is automatically printed to aid the examiner in diagnosing patients' possible illnesses.
The equipment consists of : a recording device for high-frequency, accurate eye movement measurement, two screens, an introductory computer application for behavioural testing, and an automatic test analysis application. When an eye movement exam is conducted, the tests are displayed on a screen. The subject receives instructions on how to take the tests. The subject's eye movements are recorded while he takes the test. Once the tests are completed, the examiner may conduct a thoroughly automatic analysis, with the option of manually adjusting the results to enhance quality. Based on this analysis, an eye movement report is automatically printed to aid the examiner in diagnosing patients' possible illnesses. THE RISK OF COMPULSIONS IN PARKINSON'S DISEASE
Dopamine agonist treatment of Parkinson's Disease carries a greatly increased risk of compulsive behaviour. Compulsive behaviour provoked by dopamine agonists often goes undetected in clinical series, especially if they are not specifically enquired about. Of those people with Parkinson's Disease taking dopamine agonists 22% experienced compulsive behaviour, and 16% were pathologic. However, when the analysis was restricted to patients taking dopamine agonist doses that were at least minimally therapeutic, pathological behaviours were documented in 24% of people. The most common form of compulsions caused by dopamine agonists are : gambling (36%), hypersexuality (35%), compulsive spending or shopping (26%), binge eating (17%), compulsive hobbying (12%) and compulsive computer use (9%). The vast majority of affected cases (94%) were concurrently taking Sinemet or the equivalent. Among those with adequate follow up, compulsive behaviours completely or partly resolved when the dopamine agonist dose was reduced or ceased.
DIABETICS INCREASED RISK OF PARKINSON'S DISEASE
DIABETICS INCREASED RISK OF PARKINSON'S DISEASE
 Researcher's have found that there is an increased risk of Parkinson's Disease amongst diabetics. In a study involving over a quarter of a million people the risk of Parkinson's Disease amongst diabetics was increased by 40%. Further analysis showed that the increased risk was largely limited to people who had diabetes for more than 10 years at the time of the survey. In those people the risk of developing Parkinson's Disease was increased even more, by 75%. What the researchers have not explained is why this likelihood occurs. The biochemistry of diabetes and Parkinson's Disease are quite distinct. However, both diabetes and Parkinson's Disease increase in prevalence with age.
Researcher's have found that there is an increased risk of Parkinson's Disease amongst diabetics. In a study involving over a quarter of a million people the risk of Parkinson's Disease amongst diabetics was increased by 40%. Further analysis showed that the increased risk was largely limited to people who had diabetes for more than 10 years at the time of the survey. In those people the risk of developing Parkinson's Disease was increased even more, by 75%. What the researchers have not explained is why this likelihood occurs. The biochemistry of diabetes and Parkinson's Disease are quite distinct. However, both diabetes and Parkinson's Disease increase in prevalence with age.HIGH PREVALENCE OF VITAMIN D DEFICIENCY IN PARKINSON'S DISEASE
Vitamin D insufficiency has been reported to be far more common in people with Parkinson's Disease, but it is not clear whether having a chronic disease causing reduced mobility contributes to this relatively high prevalence. Nearly 70% of people with early Parkinson's Disease have an insufficiency of vitamin D. The prevalence of vitamin D insufficiency in people with early Parkinson's Disease was similar to or higher than those reported in previous studies. Vitamin D concentrations did not decline with the worsening of Parkinson's Disease. People with Parkinson's Disease were also previously found to be prone to Osteoporosis, which is a bone disorder related to vitamin D deficiency.
 The researchers offer no explanation as to why vitamin D deficiency is so high in early Parkinson's Disease. Vitamin D is not essential for the formation of dopamine, the substance whose deficiency causes Parkinson's Disease. Vitamin D is obtained from sunshine, but can be more readily obtained in vitamin and mineral supplements, many of which include sufficient vitamin D to prevent a deficiency of vitamin D from occurring.
The researchers offer no explanation as to why vitamin D deficiency is so high in early Parkinson's Disease. Vitamin D is not essential for the formation of dopamine, the substance whose deficiency causes Parkinson's Disease. Vitamin D is obtained from sunshine, but can be more readily obtained in vitamin and mineral supplements, many of which include sufficient vitamin D to prevent a deficiency of vitamin D from occurring.
