http://health.einnews.com/article/265757930/DPMY8cLhvbBrlR9w
http://health.einnews.com/article/265757930/DPMY8cLhvbBrlR9w
WELCOME TO OUR PARKINSON'S PLACE!
I HAVE PARKINSON'S DISEASES AND THOUGHT IT WOULD BE NICE TO HAVE A PLACE WHERE THE CONTENTS OF UPDATED NEWS IS FOUND IN ONE PLACE. THAT IS WHY I BEGAN THIS BLOG.
I COPY NEWS ARTICLES PERTAINING TO RESEARCH, NEWS AND INFORMATION FOR PARKINSON'S DISEASE, DEMENTIA, THE BRAIN, DEPRESSION AND PARKINSON'S WITH DYSTONIA. I ALSO POST ABOUT FUNDRAISING FOR PARKINSON'S DISEASE AND EVENTS. I TRY TO BE UP-TO-DATE AS POSSIBLE.
I AM NOT RESPONSIBLE FOR IT'S CONTENTS. I AM JUST A COPIER OF INFORMATION SEARCHED ON THE COMPUTER. PLEASE UNDERSTAND THE COPIES ARE JUST THAT, COPIES AND AT TIMES, I AM UNABLE TO ENLARGE THE WORDING OR KEEP IT UNIFORMED AS I WISH. IT IS IMPORTANT TO UNDERSTAND I AM A PERSON WITH PARKINSON'S DISEASE. I HAVE NO MEDICAL EDUCATION,
I JUST WANT TO SHARE WITH YOU WHAT I READ ON THE INTERNET. IT IS UP TO YOU TO DECIDE WHETHER TO READ IT AND TALK IT OVER WITH YOUR DOCTOR. I AM JUST THE COPIER OF DOCUMENTS FROM THE COMPUTER. I DO NOT HAVE PROOF OF FACT OR FICTION OF THE ARTICLE. I ALSO TRY TO PLACE A LINK AT THE BOTTOM OF EACH ARTICLE TO SHOW WHERE I RECEIVED THE INFORMATION SO THAT YOU MAY WANT TO VISIT THEIR SITE.
THIS IS FOR YOU TO READ AND TO ALWAYS KEEP AN OPEN MIND.
PLEASE DISCUSS THIS WITH YOUR DOCTOR, SHOULD YOU HAVE ANY QUESTIONS, OR CONCERNS. NEVER DO ANYTHING WITHOUT TALKING TO YOUR DOCTOR FIRST..
I DO NOT MAKE ANY MONEY FROM THIS WEBSITE. I VOLUNTEER MY TIME TO HELP ALL OF US TO BE INFORMED.
I WILL NOT ACCEPT ANY ADVERTISEMENT OR HEALING POWERS, HEALING FROM HERBS AND ETC. UNLESS IT HAS GONE THROUGH TRIALS AND APPROVED BY FDA. IT WILL GO INTO SPAM.
THIS IS A FREE SITE FOR ALL WITH NO ADVERTISEMENTS
THANK YOU FOR VISITING! TOGETHER WE CAN MAKE A DIFFERENCE!

Investor and Media Contact:
Jenene Thomas
Jenene Thomas Communications, LLC
Investor Relations and Corporate Communications Advisor
T: (US) 908.938.1475E:
- See more at: http://globenewswire.com/news-release/2015/05/15/736287/10134645/en/Amarantus-Announces-Successful-Delivery-and-Distribution-of-MANF-in-Preclinical-Model-to-Brain-Areas-Involved-in-Parkinson-s-Disease.html?f=22&fvtc=3&fvtv=4000#sthash.vEQmLOOU.dpuf


 One of the biggest reasons for hospitalizations in the Parkinson’s community aside from medical emergencies and trauma (usually because medications not well adjusted) in my experience is acute side effects such as hallucinations, confusion, psychosis, or pain all stemming from improper intake of prescribed Parkinson’s medications.
One of the biggest reasons for hospitalizations in the Parkinson’s community aside from medical emergencies and trauma (usually because medications not well adjusted) in my experience is acute side effects such as hallucinations, confusion, psychosis, or pain all stemming from improper intake of prescribed Parkinson’s medications.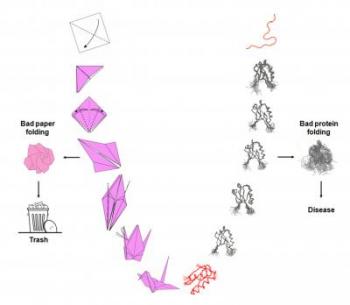
CONTACT: Investor and Media Contact:
Jenene Thomas
Jenene Thomas Communications, LLC
Investor Relations and Corporate Communications Advisor
T: (US) 908.938.1475
E: jenene@jenenethomascommunications.com
http://health.einnews.com/article/265229520/rvyFoJ2KUK4cAsJu
Researchers say more work needs to be done to understand findings
By Robert Preidt
|