 Every one of the seven generic versions of Madopar had one or two parameters outside the specifications of Madopar. Deviations for the active ingredients ranged from 8% more benserazide to 7% less L-dopa in two of the tablet formulations. Degradation products were measured in marked excess (26% more) in one capsule formulation, and so could pose a safety concern. Deviations for the active ingredients may go unnoticed by a new user of the generic product but may entail clinical consequences when switching over. The results therefore suggest caution when prescribing a generic version of Madopar or any other generic.
Every one of the seven generic versions of Madopar had one or two parameters outside the specifications of Madopar. Deviations for the active ingredients ranged from 8% more benserazide to 7% less L-dopa in two of the tablet formulations. Degradation products were measured in marked excess (26% more) in one capsule formulation, and so could pose a safety concern. Deviations for the active ingredients may go unnoticed by a new user of the generic product but may entail clinical consequences when switching over. The results therefore suggest caution when prescribing a generic version of Madopar or any other generic. WELCOME TO OUR PARKINSON'S PLACE!
I HAVE PARKINSON'S DISEASES AND THOUGHT IT WOULD BE NICE TO HAVE A PLACE WHERE THE CONTENTS OF UPDATED NEWS IS FOUND IN ONE PLACE. THAT IS WHY I BEGAN THIS BLOG.
I COPY NEWS ARTICLES PERTAINING TO RESEARCH, NEWS AND INFORMATION FOR PARKINSON'S DISEASE, DEMENTIA, THE BRAIN, DEPRESSION AND PARKINSON'S WITH DYSTONIA. I ALSO POST ABOUT FUNDRAISING FOR PARKINSON'S DISEASE AND EVENTS. I TRY TO BE UP-TO-DATE AS POSSIBLE.
I AM NOT RESPONSIBLE FOR IT'S CONTENTS. I AM JUST A COPIER OF INFORMATION SEARCHED ON THE COMPUTER. PLEASE UNDERSTAND THE COPIES ARE JUST THAT, COPIES AND AT TIMES, I AM UNABLE TO ENLARGE THE WORDING OR KEEP IT UNIFORMED AS I WISH. IT IS IMPORTANT TO UNDERSTAND I AM A PERSON WITH PARKINSON'S DISEASE. I HAVE NO MEDICAL EDUCATION,
I JUST WANT TO SHARE WITH YOU WHAT I READ ON THE INTERNET. IT IS UP TO YOU TO DECIDE WHETHER TO READ IT AND TALK IT OVER WITH YOUR DOCTOR. I AM JUST THE COPIER OF DOCUMENTS FROM THE COMPUTER. I DO NOT HAVE PROOF OF FACT OR FICTION OF THE ARTICLE. I ALSO TRY TO PLACE A LINK AT THE BOTTOM OF EACH ARTICLE TO SHOW WHERE I RECEIVED THE INFORMATION SO THAT YOU MAY WANT TO VISIT THEIR SITE.
THIS IS FOR YOU TO READ AND TO ALWAYS KEEP AN OPEN MIND.
PLEASE DISCUSS THIS WITH YOUR DOCTOR, SHOULD YOU HAVE ANY QUESTIONS, OR CONCERNS. NEVER DO ANYTHING WITHOUT TALKING TO YOUR DOCTOR FIRST..
I DO NOT MAKE ANY MONEY FROM THIS WEBSITE. I VOLUNTEER MY TIME TO HELP ALL OF US TO BE INFORMED.
I WILL NOT ACCEPT ANY ADVERTISEMENT OR HEALING POWERS, HEALING FROM HERBS AND ETC. UNLESS IT HAS GONE THROUGH TRIALS AND APPROVED BY FDA. IT WILL GO INTO SPAM.
THIS IS A FREE SITE FOR ALL WITH NO ADVERTISEMENTS
THANK YOU FOR VISITING! TOGETHER WE CAN MAKE A DIFFERENCE!
TRANSLATE
Friday, May 3, 2013
IS GENERIC MADOPAR AS GOOD AS MADOPAR ?
 Every one of the seven generic versions of Madopar had one or two parameters outside the specifications of Madopar. Deviations for the active ingredients ranged from 8% more benserazide to 7% less L-dopa in two of the tablet formulations. Degradation products were measured in marked excess (26% more) in one capsule formulation, and so could pose a safety concern. Deviations for the active ingredients may go unnoticed by a new user of the generic product but may entail clinical consequences when switching over. The results therefore suggest caution when prescribing a generic version of Madopar or any other generic.
Every one of the seven generic versions of Madopar had one or two parameters outside the specifications of Madopar. Deviations for the active ingredients ranged from 8% more benserazide to 7% less L-dopa in two of the tablet formulations. Degradation products were measured in marked excess (26% more) in one capsule formulation, and so could pose a safety concern. Deviations for the active ingredients may go unnoticed by a new user of the generic product but may entail clinical consequences when switching over. The results therefore suggest caution when prescribing a generic version of Madopar or any other generic. HEAD INJURY AND THE RISK OF PARKINSON'S DISEASE
Head trauma has long been implicated as one of the causes of Parkinson's Disease. Researchers recently assessed people with Parkinson's Disease who had head trauma so serious that it had led to concussion. They conducted a sensitivity analysis to assess the influence of each study. After reviewing more than 636 article titles, 34 articles were selected for full review. In total, 22 studies were included in the assessment.
 The association of Parkinson's Disease and head trauma was 1.57 (1.35-1.83), meaning that head trauma causing concussion makes Parkinson's Disease more than one and half times more likely. So although head trauma makes Parkinson's Disease more likely it is not inevitable. Further analysis of the results might have shown that very severe head injury or certain types of head injury were largely responsible for the increased likelihood of Parkinson's Disease following head trauma.
The association of Parkinson's Disease and head trauma was 1.57 (1.35-1.83), meaning that head trauma causing concussion makes Parkinson's Disease more than one and half times more likely. So although head trauma makes Parkinson's Disease more likely it is not inevitable. Further analysis of the results might have shown that very severe head injury or certain types of head injury were largely responsible for the increased likelihood of Parkinson's Disease following head trauma.HANDBOOK OF PARKINSON'S DISEASE (Fifth edition)
Rajesh Pahwa (Editor), Kelly E. Lyons (Editor)
 Publisher's description : This volume has long prevailed as one of the leading resources on Parkinson's disease. Fully updated with practical chapters on pathology, neurochemistry, etiology, and breakthrough research, it spans every essential topic related to the identification, assessment, and treatment of PD. Reflecting the many advances that have taken place in the management of PD, this volume promotes a multidisciplinary approach to care and supplies new sections on the latest pharmacologic, surgical, and rehabilitative therapies, as well as essential diagnostic, imaging, and nonmotor management strategies. New to this edition : Early identification of premotor symptoms, Potential disease modification agents, Physical and occupational therapy
Publisher's description : This volume has long prevailed as one of the leading resources on Parkinson's disease. Fully updated with practical chapters on pathology, neurochemistry, etiology, and breakthrough research, it spans every essential topic related to the identification, assessment, and treatment of PD. Reflecting the many advances that have taken place in the management of PD, this volume promotes a multidisciplinary approach to care and supplies new sections on the latest pharmacologic, surgical, and rehabilitative therapies, as well as essential diagnostic, imaging, and nonmotor management strategies. New to this edition : Early identification of premotor symptoms, Potential disease modification agents, Physical and occupational therapyNEURTURIN FAILS CLINICAL TRIALS FOR PARKINSON'S DISEASE
 The clinical trial did not demonstrate statistically significant efficacy. Yet, following surgery, there was a marked placebo effect in those people being tested and even those not being tested, as there often is after surgical trials. The clinical trial was supported by the Michael J.Fox Foundation. The results suggest that it is unclear if Ceregene will move forward with the development of CERE-120 as a viable treatment for people with Parkinson’s Disease.
The clinical trial did not demonstrate statistically significant efficacy. Yet, following surgery, there was a marked placebo effect in those people being tested and even those not being tested, as there often is after surgical trials. The clinical trial was supported by the Michael J.Fox Foundation. The results suggest that it is unclear if Ceregene will move forward with the development of CERE-120 as a viable treatment for people with Parkinson’s Disease. BRAIN CELL LOSS IN PARKINSON'S DISEASE
 For many years it has been widely claimed that, in Parkinson's Disease, there is a huge loss of the dopaminergic neurons (the brain cells that produce dopamine). It is often claimed that this cell loss is the primary cause of Parkinson's Disease. However, not a single study had ever actually shown that there was massive cell loss in Parkinson's Disease. It has also been assumed that loss of the dopaminergic neurons that can cause Parkinson's Disease precedes the loss of cholinergic neurons, which can lead to dementia, as is common in later Parkinson's Disease. However, the results of a study assessing this theory did not support what had often been claimed.
For many years it has been widely claimed that, in Parkinson's Disease, there is a huge loss of the dopaminergic neurons (the brain cells that produce dopamine). It is often claimed that this cell loss is the primary cause of Parkinson's Disease. However, not a single study had ever actually shown that there was massive cell loss in Parkinson's Disease. It has also been assumed that loss of the dopaminergic neurons that can cause Parkinson's Disease precedes the loss of cholinergic neurons, which can lead to dementia, as is common in later Parkinson's Disease. However, the results of a study assessing this theory did not support what had often been claimed.DEFYING DEMENTIA
Kevin Davies
 Publisher's description : As we get older there are few things more frightening than forgetting someone's name or where you left your house keys. For millions of us the first few times this happens we fear we're experiencing the onset of dementia. Recognizing the symptoms can lead to an early diagnosis which can mean better treatment options, a longer, better life and a change to take steps to slow or reduce the symptoms of dementia. There are many drugs and treatments the patient needs to retrain parts of their brain that have been damaged. You can DEFY it by learning the symptoms, getting diagnosed early, and getting treatment quickly. Diagnosis only takes 10 minutes. Ten minutes is all that stands between you and knowing if your lapse of memory is fatigue, a vitamin B deficiency, some other curable disease, or depression.
Publisher's description : As we get older there are few things more frightening than forgetting someone's name or where you left your house keys. For millions of us the first few times this happens we fear we're experiencing the onset of dementia. Recognizing the symptoms can lead to an early diagnosis which can mean better treatment options, a longer, better life and a change to take steps to slow or reduce the symptoms of dementia. There are many drugs and treatments the patient needs to retrain parts of their brain that have been damaged. You can DEFY it by learning the symptoms, getting diagnosed early, and getting treatment quickly. Diagnosis only takes 10 minutes. Ten minutes is all that stands between you and knowing if your lapse of memory is fatigue, a vitamin B deficiency, some other curable disease, or depression. THE PARKINSON HUB New web site
THE PARKINSON HUB
 Theparkinsonhub is a recent Parkinson's Disease web site that aims to provide patients, carers and healthcare professionals with the latest news, link and information in the area of Parkinson’s disease. For more information, go to theparkinsonhub. They have just introduced the complementary PD Quality of Life interactive resource, which enables people with Parkinson's Disease to identify and understand those areas in their lives most affected by Parkinson's Disease
Theparkinsonhub is a recent Parkinson's Disease web site that aims to provide patients, carers and healthcare professionals with the latest news, link and information in the area of Parkinson’s disease. For more information, go to theparkinsonhub. They have just introduced the complementary PD Quality of Life interactive resource, which enables people with Parkinson's Disease to identify and understand those areas in their lives most affected by Parkinson's DiseaseISTRADEFYLLINE CLINICAL TRIAL RESULTS FOR PARKINSON'S DISEASE
Researchers evaluated the efficacy and safety of istradefylline, which is being developed for the treatment of Parkinson's Disease. Istradefylline is an A(2A) adenosine receptor antagonist and so does not act by directly increasing the activity of dopamine. It is administered with L-dopa.
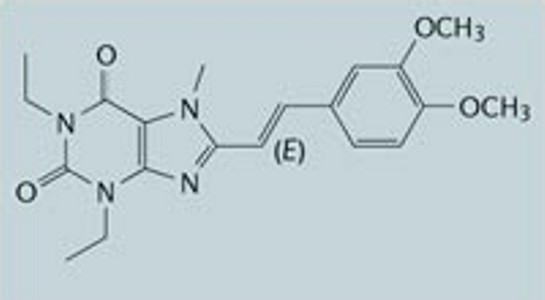 After a 12 week clinical trial using 20mg or 40mg istradefylline the change in daily OFF time was significantly reduced with 20mg per day and 40 mg per day. The daily OFF time was over 40 minutes less. However, the most common adverse event was dyskinesia, which occurred more commonly when taking istradefylline than when taking a placebo
After a 12 week clinical trial using 20mg or 40mg istradefylline the change in daily OFF time was significantly reduced with 20mg per day and 40 mg per day. The daily OFF time was over 40 minutes less. However, the most common adverse event was dyskinesia, which occurred more commonly when taking istradefylline than when taking a placeboINHALED DOPAMINE AGONIST CLINICAL TRIAL RESULTS
Researchers assessed the safety, tolerability and efficacy of a new form of apomorphine presently being developed, called VR040, which is an inhaled dry powder. Apomorphine is a dopamine agonist used in the treatment of Parkinson's Disease. 'Off' periods usually increase as Parkinson's Disease progresses and the benefits of standard therapy wane. Subcutaneous (injected) apomorphine rescues 'off' periods, but injections by patients and adverse effects are sometimes problematic.
 Inhaled doses were gradually increased until efficacy was reached and given to patients when they were in an 'off' state. When it was inhaled, apomorphine was rapidly absorbed, within 2 to 7 minutes. This enabled a reversal from the 'off' state, in just 10 minutes. In contrast many people with Parkinson's Disease have to wait 30 to 60 minutes for their Parkinson's Disease drugs to have effect. Therefore, speed of effect appears to be its greatest benefit. Adverse effects did not differ between those taking inhaled apomorphine and those taking a placebo.
Inhaled doses were gradually increased until efficacy was reached and given to patients when they were in an 'off' state. When it was inhaled, apomorphine was rapidly absorbed, within 2 to 7 minutes. This enabled a reversal from the 'off' state, in just 10 minutes. In contrast many people with Parkinson's Disease have to wait 30 to 60 minutes for their Parkinson's Disease drugs to have effect. Therefore, speed of effect appears to be its greatest benefit. Adverse effects did not differ between those taking inhaled apomorphine and those taking a placebo.PARKINSON'S TREATMENT : 10 SECRETS TO A HAPPIER LIFE
PARKINSON'S TREATMENT : 10 SECRETS TO A HAPPIER LIFE
Michael S.Okun MD
 Publisher's description : I never assume a sufferer or family member is aware of the “secrets” that may lead to hope and to a happier life. We must share these secrets, and this is the purpose of this book. Each chapter of this book reveals an important secret, and with each secret I will explain the insight, the rationale, the empiricism, and the science behind it. In each chapter I will also try to reveal a little more about myself, and a lot more about the patients and talented clinicians who gifted the Parkinson's secrets. These patients planted the seed of faith. They learned to grow hope, and they discovered the core values necessary to achieve happiness despite the chronic illness of Parkinson's disease
Publisher's description : I never assume a sufferer or family member is aware of the “secrets” that may lead to hope and to a happier life. We must share these secrets, and this is the purpose of this book. Each chapter of this book reveals an important secret, and with each secret I will explain the insight, the rationale, the empiricism, and the science behind it. In each chapter I will also try to reveal a little more about myself, and a lot more about the patients and talented clinicians who gifted the Parkinson's secrets. These patients planted the seed of faith. They learned to grow hope, and they discovered the core values necessary to achieve happiness despite the chronic illness of Parkinson's disease People with Parkinson's Disease were given two forms of ODM-101 with two different amounts of carbidopa : ODM-101/65mg and ODM-101/105mg. Both of them reduced daily OFF-time more than Stalevo. ODM-101/105mg was marginally better than ODM-101/65mg. Similarly, both ODM-101 combinations increased the ON-time without troublesome dyskinesia significantly more than Stalevo. There were no significant differences between the treatments in ON-time with troublesome dyskinesia or in UPDRS II and III symptom scores. Overall, tolerability and safety of ODM-101 was comparable to that of Stalevo.
People with Parkinson's Disease were given two forms of ODM-101 with two different amounts of carbidopa : ODM-101/65mg and ODM-101/105mg. Both of them reduced daily OFF-time more than Stalevo. ODM-101/105mg was marginally better than ODM-101/65mg. Similarly, both ODM-101 combinations increased the ON-time without troublesome dyskinesia significantly more than Stalevo. There were no significant differences between the treatments in ON-time with troublesome dyskinesia or in UPDRS II and III symptom scores. Overall, tolerability and safety of ODM-101 was comparable to that of Stalevo.PIMAVANSERIN CLINICAL TRIAL RESULTS IN PARKINSON'S DISEASE
 Patients took 40mg Pimavanserin for only six weeks. So its long term effects have not been assessed. The adverse events were little different from those found in people taking a placebo. Pimavanserin demonstrated significant antipsychotic efficacy and significant improvements in all secondary efficacy measures. Statistically significant benefits were also observed in exploratory measures of nighttime sleep, daytime wakefulness, and caregiver burden.
Patients took 40mg Pimavanserin for only six weeks. So its long term effects have not been assessed. The adverse events were little different from those found in people taking a placebo. Pimavanserin demonstrated significant antipsychotic efficacy and significant improvements in all secondary efficacy measures. Statistically significant benefits were also observed in exploratory measures of nighttime sleep, daytime wakefulness, and caregiver burden.BEING OVERWEIGHT IS MORE PREVALENT IN PARKINSON'S DISEASE
 Underweight and malnutrition are well documented in Parkinson's Disease (PD), while being overweight has been less reported. Researchers carried out a study comparing the weight and height of people with and without Parkinson's Disease. In those people with Parkinson's Disease only 1% were underweight, 33% were within the normal range, 47%% were overweight, and 19% were obese. Being normal weight and overweight were more prevalent in those people who had Parkinson's Disease when compared to those people who did not. Being obese and, even moreso, being underweight were more common in those people who did not have Parkinsons' Disease.
Underweight and malnutrition are well documented in Parkinson's Disease (PD), while being overweight has been less reported. Researchers carried out a study comparing the weight and height of people with and without Parkinson's Disease. In those people with Parkinson's Disease only 1% were underweight, 33% were within the normal range, 47%% were overweight, and 19% were obese. Being normal weight and overweight were more prevalent in those people who had Parkinson's Disease when compared to those people who did not. Being obese and, even moreso, being underweight were more common in those people who did not have Parkinsons' Disease.CLINICAL TRIAL OF SUBCUTANEOUS L-DOPA FOR PARKINSON'S DISEASE
 NeuroDerm announced today the results of a Phase I safety and pharmacokinetic trial of ND0612. ND0612 is a novel drug formulation for the treatment of Parkinson’s Disease. NeuroDerm is a pharmaceutical company that specializes in the development of novel dermal delivery routes for CNS drugs.
NeuroDerm announced today the results of a Phase I safety and pharmacokinetic trial of ND0612. ND0612 is a novel drug formulation for the treatment of Parkinson’s Disease. NeuroDerm is a pharmaceutical company that specializes in the development of novel dermal delivery routes for CNS drugs. HOW TO LIVE WELL WITH PARKINSON'S : ADVICE FROM A PHYSICAL THERAPIST
HOW TO LIVE WELL WITH PARKINSON'S : ADVICE FROM A PHYSICAL THERAPIST
Miriam P. Boelen
 Publisher's description : People with Parkinson’s commonly have symptoms and problems unique to their condition that can interfere with daily activities. When initially diagnosed they all too often don’t know what to do or where to turn. This book, written specifically for them, clears up questions they may have regarding their self-help. It gives step by step instructions in properly handling daily activities like walking, getting out of bed or chairs, and other potentially problematic everyday movements. It also guides them in finding the optimal medical team to help them stay well. For caregivers there are easy-to-follow instructions in safely assisting a person with PD without jeopardizing themselves or the one they are helping
Publisher's description : People with Parkinson’s commonly have symptoms and problems unique to their condition that can interfere with daily activities. When initially diagnosed they all too often don’t know what to do or where to turn. This book, written specifically for them, clears up questions they may have regarding their self-help. It gives step by step instructions in properly handling daily activities like walking, getting out of bed or chairs, and other potentially problematic everyday movements. It also guides them in finding the optimal medical team to help them stay well. For caregivers there are easy-to-follow instructions in safely assisting a person with PD without jeopardizing themselves or the one they are helping
