May 13,2015
You are taking a class on origami and Mr. Otaki asks you to fold that little red piece of paper into a very elaborate design. You have to do it in a very short time. You try your best but you fail. Your origami sucks!
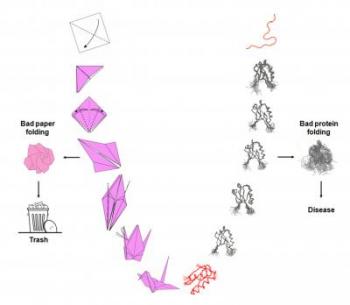
Bad protein folding is associated with a number of age-related diseases including Parkinson's, Alzheimer's, Huntington's and even cancer.
Credit: Guilherme Augusto
Surprised, Mr. Otaki wants to understand what you did wrong and starts unfolding your failed attempt at a red dragon. The unfolding reveals all the intermediate steps you went through and indicates exactly how you screwed up. That attempt at a red dragon is now history and lies in the trash basket. You get another square and prepare for a fresh attempt at mastering the art of paper folding.
Protein folding is the process by which a protein undergoes many folding steps until it assumes its functional conformation, a three-dimensional structure called the native state of the protein. It is in this native state that proteins are able to do the many jobs assigned to them, including cell division, growth, cell nutrition and locomotion, among others. The story of protein folding is not unrelated to origami--except that unlike bad paper folding, which simply goes in the trash, bad protein folding is associated with a number of age-related diseases including Parkinson's, Alzheimer's, Huntington's and even cancer.
It is already known that protein misfolding generates a number of intermediate proteins that may accumulate in the brain. For instance, an abnormally folded version of the protein amyloid beta accumulates in the brain of Alzheimer's patients, damaging neurons and impairing the brain in carrying out its normal functions. Thus, a good understanding of protein folding and misfolding might be crucial for the development of treatments against these devastating diseases.
Researchers have used physical and chemical strategies such as high pressure and urea to force folded proteins into unfolding so all the steps, and the intermediate proteins that are generated along the process, can be observed and studied. However, the mechanisms underlying the effects of these two strategies and how exactly they work in protein unfolding have never been clearly understood.
Using nuclear magnetic resonance spectroscopy, a technique that provides detailed information on the structure, composition, state, and chemical environment of molecules, and small-angle X-ray scattering, which informs on the shape and size of macromolecules, Dr. Jerson Lima Silva and Dr. Guilherme Augusto, at the Federal University of Rio de Janeiro, Brazil, have revealed for the first time all the mechanistic details on how high pressure and urea act on proteins. "We were surprised to see that these two strategies differ significantly in the way they force proteins into unfolding," says Dr. Silva.
In a paper entitled "A hypothesis to reconcile the physical and chemical unfolding of proteins" and published in the renowned journal Proceedings of the National Academy of Sciences in the week of May 11, 2015, the two authors show that whereas high pressure pushes water molecules towards the protein until it unfolds, urea has the opposite effect by pulling the protein's molecules. Although the final result seems similar, with the protein unfolding in both cases, the intermediates formed are very different and the roads to unfolding are very specific.
"The push-and-pull hypothesis predicts that the molecular mechanisms leading to protein unfolding can be very different depending on the nature of the causal agent involved," says Dr. Guilherme Augusto.
These new findings greatly contribute to our understanding on how proteins' native state might be disrupted, and hopefully will open effective new avenues for how to avoid such disruption.


No comments:
Post a Comment