 Researchers assessed
whether it would be suitable to use Subthalamic stimulation at an earlier stage
of Parkinson's Disease. Subthalamic stimulation, which is referred to as DBS
(Deep Brain Stimulation), involves the use of
electrodes that are implanted into the brain and connected to a small electrical
device called a pulse generator that can be externally programmed. DBS can
reduce the need for L-dopa and related drugs, which in turn decreases the
involuntary movements called dyskinesias that are a common side effect of
L-dopa.
Researchers assessed
whether it would be suitable to use Subthalamic stimulation at an earlier stage
of Parkinson's Disease. Subthalamic stimulation, which is referred to as DBS
(Deep Brain Stimulation), involves the use of
electrodes that are implanted into the brain and connected to a small electrical
device called a pulse generator that can be externally programmed. DBS can
reduce the need for L-dopa and related drugs, which in turn decreases the
involuntary movements called dyskinesias that are a common side effect of
L-dopa. WELCOME TO OUR PARKINSON'S PLACE!
I HAVE PARKINSON'S DISEASES AND THOUGHT IT WOULD BE NICE TO HAVE A PLACE WHERE THE CONTENTS OF UPDATED NEWS IS FOUND IN ONE PLACE. THAT IS WHY I BEGAN THIS BLOG.
I COPY NEWS ARTICLES PERTAINING TO RESEARCH, NEWS AND INFORMATION FOR PARKINSON'S DISEASE, DEMENTIA, THE BRAIN, DEPRESSION AND PARKINSON'S WITH DYSTONIA. I ALSO POST ABOUT FUNDRAISING FOR PARKINSON'S DISEASE AND EVENTS. I TRY TO BE UP-TO-DATE AS POSSIBLE.
I AM NOT RESPONSIBLE FOR IT'S CONTENTS. I AM JUST A COPIER OF INFORMATION SEARCHED ON THE COMPUTER. PLEASE UNDERSTAND THE COPIES ARE JUST THAT, COPIES AND AT TIMES, I AM UNABLE TO ENLARGE THE WORDING OR KEEP IT UNIFORMED AS I WISH. IT IS IMPORTANT TO UNDERSTAND I AM A PERSON WITH PARKINSON'S DISEASE. I HAVE NO MEDICAL EDUCATION,
I JUST WANT TO SHARE WITH YOU WHAT I READ ON THE INTERNET. IT IS UP TO YOU TO DECIDE WHETHER TO READ IT AND TALK IT OVER WITH YOUR DOCTOR. I AM JUST THE COPIER OF DOCUMENTS FROM THE COMPUTER. I DO NOT HAVE PROOF OF FACT OR FICTION OF THE ARTICLE. I ALSO TRY TO PLACE A LINK AT THE BOTTOM OF EACH ARTICLE TO SHOW WHERE I RECEIVED THE INFORMATION SO THAT YOU MAY WANT TO VISIT THEIR SITE.
THIS IS FOR YOU TO READ AND TO ALWAYS KEEP AN OPEN MIND.
PLEASE DISCUSS THIS WITH YOUR DOCTOR, SHOULD YOU HAVE ANY QUESTIONS, OR CONCERNS. NEVER DO ANYTHING WITHOUT TALKING TO YOUR DOCTOR FIRST..
I DO NOT MAKE ANY MONEY FROM THIS WEBSITE. I VOLUNTEER MY TIME TO HELP ALL OF US TO BE INFORMED.
I WILL NOT ACCEPT ANY ADVERTISEMENT OR HEALING POWERS, HEALING FROM HERBS AND ETC. UNLESS IT HAS GONE THROUGH TRIALS AND APPROVED BY FDA. IT WILL GO INTO SPAM.
THIS IS A FREE SITE FOR ALL WITH NO ADVERTISEMENTS
THANK YOU FOR VISITING! TOGETHER WE CAN MAKE A DIFFERENCE!
TRANSLATE
Wednesday, March 20, 2013
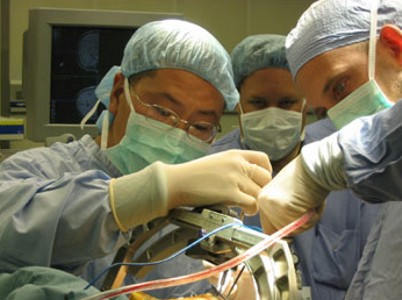
DBS IS EFFECTIVE IN EARLIER PARKINSON'S DISEASE
 Researchers assessed
whether it would be suitable to use Subthalamic stimulation at an earlier stage
of Parkinson's Disease. Subthalamic stimulation, which is referred to as DBS
(Deep Brain Stimulation), involves the use of
electrodes that are implanted into the brain and connected to a small electrical
device called a pulse generator that can be externally programmed. DBS can
reduce the need for L-dopa and related drugs, which in turn decreases the
involuntary movements called dyskinesias that are a common side effect of
L-dopa.
Researchers assessed
whether it would be suitable to use Subthalamic stimulation at an earlier stage
of Parkinson's Disease. Subthalamic stimulation, which is referred to as DBS
(Deep Brain Stimulation), involves the use of
electrodes that are implanted into the brain and connected to a small electrical
device called a pulse generator that can be externally programmed. DBS can
reduce the need for L-dopa and related drugs, which in turn decreases the
involuntary movements called dyskinesias that are a common side effect of
L-dopa. 
No comments:
Post a Comment